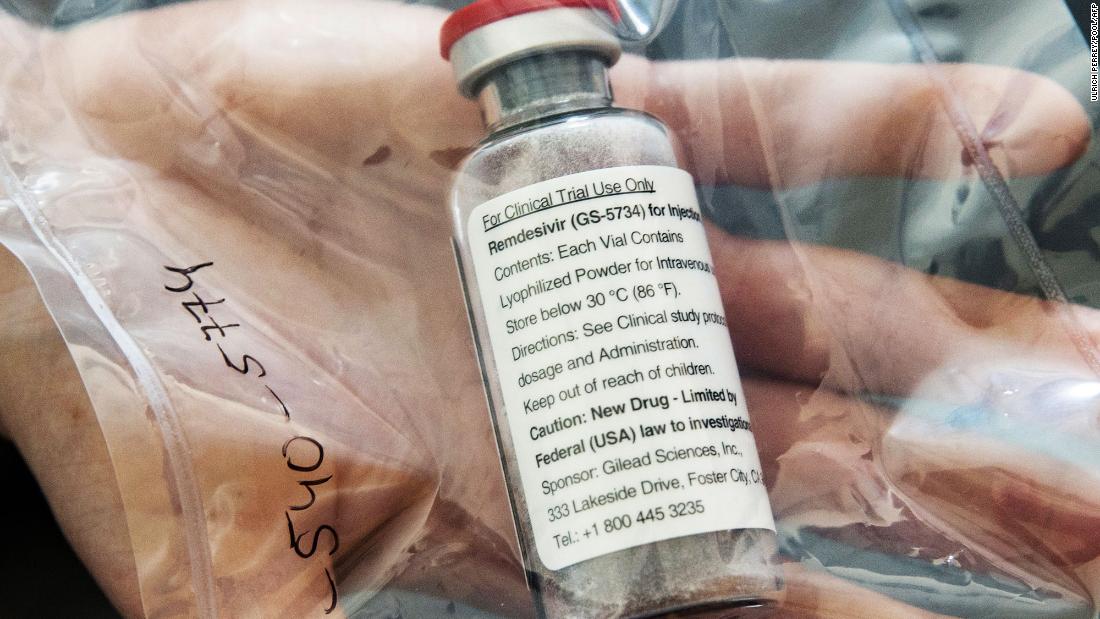
Remdesivir trial shared prematurely was ‘inconclusive,’ Gilead says
The information is no longer on WHO’s website.
“The study was terminated early due to low enrollment and, as a result, it was underpowered to enable statistically meaningful conclusions,” the tweet said. “As such, the study results are inconclusive, though trends in the data suggest a potential benefit for remdesivir, particularly among patients treated early in disease. We understand the available data have been submitted for peer-reviewed publication, which will provide more detailed information from this study in the near future.”
CNN has reached out to WHO for comment.
Gilead’s statement on Thursday said there are multiple ongoing Phase III studies designed to help gather additional data needed to determine remdesivir’s potential as a Covid-19 treatment.
“These studies will help inform whom to treat, when to treat and how long to treat with remdesivir,” the statement said. “The studies are either fully enrolled for the primary analysis or on track to fully enroll in the near future.”
Currently, there is no treatment for Covid-19 approved by the US Food and Drug Administration. While doctors are trying various treatments, it’s not yet known if they’ll work.
The FDA has said that 72 active trials are underway, with another 211 in the planning stages as of April 19.
![]()