First pill to treat Covid-19 approved in the UK: Molnupiravir
UK becomes first country in world to approve Merck’s at-home pill for Covid that HALVES risk of severe illness in vulnerable and elderly patients
Antiviral molnupiravir is first at-home antiviral proven to treat Covid patientsTablet given twice a day to Covid-positive people and will be sold as LagevrioTargeted at those deemed high risk, including over-60s and vulnerable adults
<!–
<!–
<!–<!–
<!–
(function (src, d, tag){
var s = d.createElement(tag), prev = d.getElementsByTagName(tag)[0];
s.src = src;
prev.parentNode.insertBefore(s, prev);
}(“https://www.dailymail.co.uk/static/gunther/1.17.0/async_bundle–.js”, document, “script”));
<!–
DM.loadCSS(“https://www.dailymail.co.uk/static/gunther/gunther-2159/video_bundle–.css”);
<!–
The first at-home pill to treat Covid has been approved by Britain’s medicines watchdog.
Antiviral molnupiravir was shown in clinical trials to slash the risk of hospitalisation by half in vulnerable and elderly patients.
The tablet – which will be sold under the brand name Lagevrio – will be given twice a day to people within a week of testing positive.
It will be targeted at those deemed high risk, including Britons over the age of 60, or patients with heart disease, diabetes, obesity, or other comorbidities.
Health secretary Sajid Javid said the treatment was a ‘gamechanger’ for the most frail and immunosuppressed, who are vulnerable to Covid even when vaccinated.
The pill is expected to be rolled out on the NHS within weeks, providing the country with an extra layer of defence heading into winter.
Britain has bought 480,000 doses of molnupiravir at a cost thought to be in the region of £250million.
Pills will be given out on the NHS to those have been vaccinated or unvaccinated alike, and officials will collect data on how effective they are in the real world before making a decision on whether to buy any more.
Announcing its approval today, the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) said the drug was safe and effective
Molnupiravir, made by pharmaceutical giant Merck and its partner Ridgeback Biotherapeutics, works by blocking the virus from replicating in the body.
A landmark study at the start of the month showed molnupiravir can cut hospitalisations and deaths by up to 50 per cent. It works by disrupting the Covid virus’s ability to reproduce in the human body
Officials did not disclose how much the drugs cost the Department of Health and Social Care (DHSC) but the US in June spent $1.2billion (£869million) on an order of 1.7million molnupiravir (left) courses. Health Secretary Sajid Javid (pictured right, outside No10 today) says he is ‘delighted to confirm we may soon have a new defence in our arsenal’
The pill works by introducing errors into Covid’s genetic code to hamper its ability to multiply in human cells.
It does this by attacking an enzyme that the virus relies on to generate copies of itself. Merck said the tablet should be able to tackle new variants as Covid evolves.
Vaccines, which are being made slightly weaker by new strains of the virus, work by targeting the spike protein and were designed to tackle the original virus.
Because a lot of evolution happens on the spike protein, the immune systems of vaccinated people sometimes find it more difficult to recognise new variants.
Officials did not disclose how much the Department of Health paid but American health chiefs spent $1.2billion (£869million) on 1.7million molnupiravir pills this summer.
If the drugs are priced the same in Britain they are likely to cost around £250million — even though they are thought to cost just £12 to make.
Mr Javid said: ‘Today is a historic day for our country, as the UK is now the first country in the world to approve an anti-viral that can be taken at home for Covid-19.
‘This will be a gamechanger for the most vulnerable and the immunosuppressed, who will soon be able to receive the ground-breaking treatment.
‘We are working at pace across the Government and with the NHS to set out plans to deploy molnupiravir to patients through a national study as soon as possible
‘This antiviral will be an excellent addition to our armoury against Covid-19, and it remains vital everyone comes forward for their life-saving Covid-19 vaccine – particularly those eligible for a booster – to ensure as many people as possible are protected over the coming months.’
The study on molnupiravir was on a group of 775 people who were unvaccinated.
So its effectiveness at reducing 50 per cent of serious disease could over or underestimate its true efficacy in a highly vaccinated population.
Health officials will monitor patients who are given the drug to see how it affects those who are jabbed.
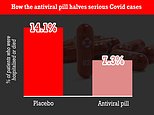
Clinical trials of the drug found that 7.3 per cent of patients given the drug were hospitalised compared to 14.1 per cent in the control group.
There were no deaths in those prescribed molnupiravir, but eight patients who were given a placebo in the trial later died of Covid.
The MHRA said the drug had been authorised for people with at least one risk factor for Covid and who have mild to moderate illness after getting infected.
The organisation’s chief executive, June Raine, described the treatment as ‘another therapeutic to add to our armoury against Covid-19’.
‘It is the world’s first approved antiviral for this disease that can be taken by mouth rather than administered intravenously,’ she said.
‘This is important, because it means it can be administered outside of a hospital setting, before Covid-19 has progressed to a severe stage.’
Former Health Secretary Matt Hancock set up the Antivirals Taskforce in April to help add to the NHS’s arsenal of treatments for Covid.
It aimed to have two new drugs available by the end of year — something which it will have failed to do unless it pens a deal for another antiviral that is available to dish out before the end of the year.
![]()